Urgent call for Londoners to take part in a new Covid-19 vaccine study
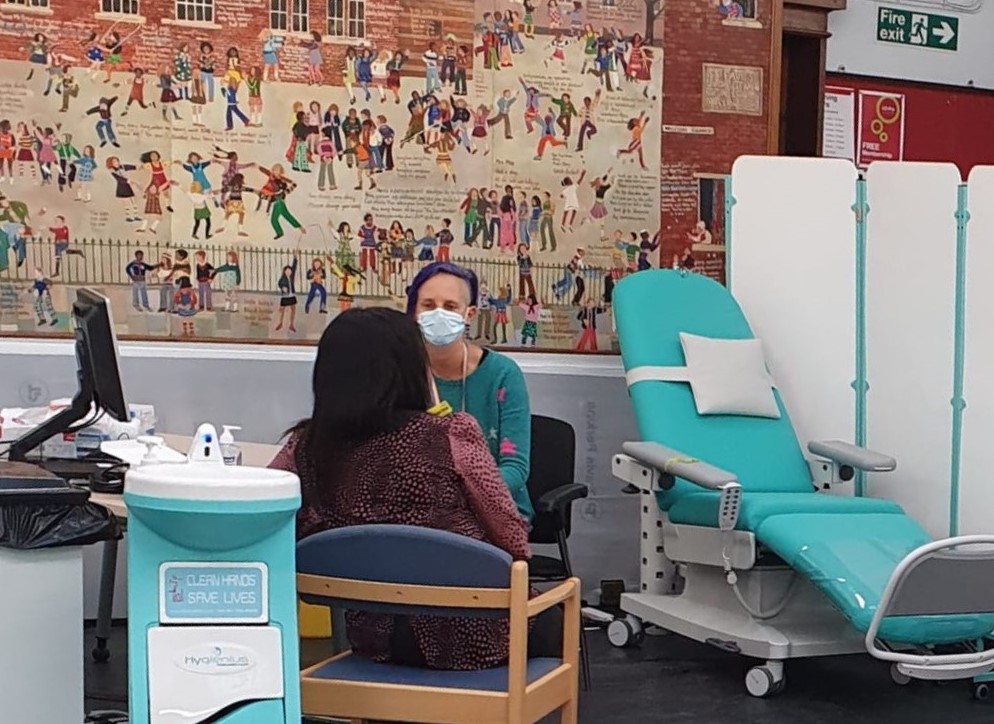
Londoners are today being urged to join a leading phase three Covid-19 vaccine study, as Barts Health NHS Trust and Queen Mary University of London administer a new trial from the Barts Health Vaccines Trials Centre at Bethnal Green Library.
The latest study, co-funded by the UK government’s Vaccine Taskforce, will test the safety and effectiveness of a new two-dose regimen for a vaccine candidate, developed by The Janssen Pharmaceutical Companies of Johnson & Johnson.
The study will recruit up to 30,000 people worldwide with 6,000 of them in the UK, and Barts Health is looking to recruit around 400 participants.
Staff from all hospital sites across Barts Health, together with Queen Mary researchers, will be working together, alongside medical school students and Trust volunteers, to run the trial at the centre.
The public are encouraged to help continue the urgent search for safe and effective Covid-19 vaccines that work for all by volunteering for this phase three trial and help ensure people in the UK have access to different types of vaccines that work for all.
Covid-19 can affect anyone and everyone, but it disproportionality affects people from Black, Asian and other ethnic minority (BAME) communities. Research has shown that people from BAME communities were more likely to die from Covid-19.
This is evident when we look at the effects of Covid-19 on east London communities, who were severely affected in the first peak of the pandemic.
The Barts Health Vaccines Trials Centre at Bethnal Green Library is therefore encouraging people from all ethnicities, minorities and health groups to take part in this trial to ensure any vaccines developed work for everyone.
Recruitment into the ENSEMBLE-2 study, which will be led by Professor Patrick Kennedy, will complete in March 2021 and the study will last for 12 months.
Professor Patrick Kennedy, principal investigator for the study and honorary hepatologist consultant at Barts Health, said:
“While the recent data from the Pfizer phase three study represents a potential major breakthrough in tackling the Covid public health emergency, we will still have to overcome significant challenges before we have a number of safe and effective vaccines which are available globally.
“More vaccine trials are needed to better understand how effective they will be in different age groups, in people with chronic medical conditions and for how long the vaccines will provide immunity.
“I am delighted to be the principal investigator on this study and to be working with an outstanding team at Barts Health so we can address these important questions and contribute to the development of a safe and effective vaccine.”
Chloe Orkin, clinical director of the Barts Health Vaccine Trials Centre and professor of HIV Medicine at Queen Mary University of London, said:
“Offering vaccine research adds to our Covid-19 treatment research which has already saved lives. I am really excited and proud to be leading this effort to offer both our staff and the people of east London this opportunity to take part in a vaccine study.
“The hope is that our participation will lead to a safe and effective vaccine for our patients, ourselves and our families and a return to normal life”
Dr Vanessa Apea, a consultant in sexual health and HIV at Barts Health, and a Black, Asian and minority ethnic clinical champion at NIHR Clinical Research Network North Thames, said:
“Covid-19 still poses a significant threat to our health and our communities and many of us are still vulnerable to it. One of the ways we can reduce the threat and impact of this disease is a vaccine.
“The topic of vaccines divides communities. For many, and in particular, Black, Asian and ethnic minority communities, the word vaccine generates a lot of fear, rooted in mistrust, which can understandably lead to reluctance in taking part in a trial.
“We know that these communities are disproportionately affected by Covid-19 and this makes it even more important that any outcomes from research, including new treatments and ways to prevent the disease, work for all communities.
“Only by doing this can we truly take control of Covid-19, so we really need people from Black, Asian and ethnic minority communities to sign up to learn more and be part of research.”
If you want to know more about taking part in this clinical trial, visit the Barts Health NHS Trust website or email covidresearch.bartshealth@nhs.net.
The UK public can support the national effort to speed up vaccine research and receive more information about volunteering for clinical studies by visiting www.nhs.uk/researchcontact to join the NHS Covid-19 Vaccine Research Registry.
